LPPVs & Local PV Services
Utilizing our Unified Governance Model, we consolidate PV operations across all covered countries into a seamless and integrated regional system. This approach allows us to provide our clients with a single interface for oversight and compliance monitoring as well as deliver a consistent service and outputs globally.
- Provision of Local Persons for Pharmacovigilance (LPPVs) and Deputies
- Creation and maintenance of local PSMF or PV System Sub-Files (PSSF)
- Assurance of 24/7 availability where required
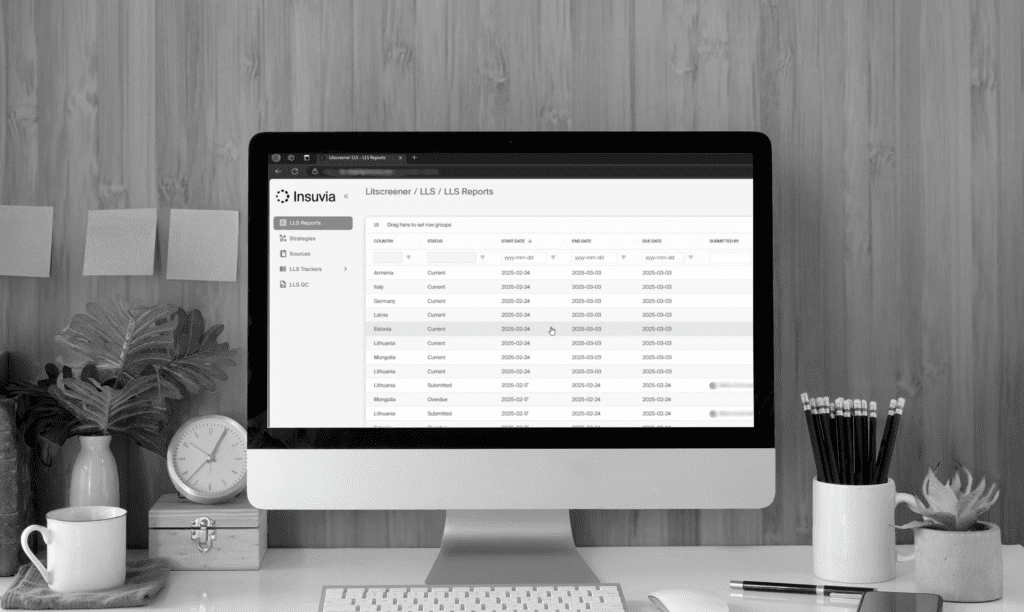
- Local Literature Screening
- Local PV regulatory intellingence/ legislation monitoring
- Handling of safety reports – intake, processing, reporting, tracking
- Local implementation of aRMMs (Educational Materials, DHPC communications)
- Submission of domestic and foreign ICSRs to local Competent Authorities where required
- Local adaptation and submission of aggregate reports (PSUR, PBRER) where required
- Local adaptation and submission of Risk Management Plans (RMPs) where required
- Local safety notifications / Signals submission
- PV training for customer’s local affiliate or distributor staff
- Handling of requests and inspections from local Competent Authorities

-
Technologies
Litscreener™: Our cutting-edge, proprietary Local Literature Monitoring System, designed to revolutionize how local literature screening is managed and executed.
RegMonitor™: Our proprietary technology for Regulatory Intelligence that ensures robust process control, higher efficiency, and superior quality.
-
Roles Fulfilled
Local Qualified Person for Pharmacovigilance (Local QPPV)
Local Safety Responsible (LSR)
Local Person for Pharmacovigilance (LPPVs)
Local Safety Officer (LSO)
Local Contact Person for Pharmacovigilance (LCPPV)
-
Regional Expertise
Europe (EU/EEA, UK)
Eurasia (EAEU & CIS)
Middle East, North & Sub-Saharan Africa
Asia Pacific
Latin America
Canada

- 60+ Countries covered
- 7+ Average years of experience of local PV personnel
- 100+ Local PV experts
-
Where are LPPVs required in EU/EEA?
Local Persons for Pharmacovigilance (LPPVs) are required in several EU member states where national regulations specify the need for a local pharmacovigilance contact. The specific requirements vary across the EU, with some countries mandating an LPPV to ensure compliance with local pharmacovigilance obligations and facilitate communication with national health authorities. For detailed information on the specific countries and requirements, visit visit this link or contact us for a free consultation.
-
How to optimize your LPPV strategy for compliance and cost-effectiveness?
When developing an LPPV strategy in the EU, it’s essential to understand where LPPVs are required, consider possible exemptions for your situation, and appoint LPPVs at the right time (e.g. product approval or launch, etc.) to avoid unnecessary costs. It’s also important to plan for Deputy person requirements and navigate language and residency rules effectively. A well-planned LPPV strategy can optimize compliance, reduce redundancy, and save resources. For more information we recommend reading our article on LPPV strategy consideration or contacting us for a free consultation.
-
In which countries and when should a MAH start local literature monitoring?
According to GVP Module 6 VI.B.1.1.2, a Marketing Authorization Holder (MAH) must monitor local publications in any country where their product has marketing authorization. Therefore, local literature monitoring should begin immediately once the product is approved in that country and must be continuously conducted in all countries where the MAH has product approvals to ensure ongoing compliance with pharmacovigilance requirements.
-
Which local publications should be monitored for pharmacovigilance?
Some regulatory authorities provide lists of journals they expect to be monitored, while others require the Marketing Authorization Holder (MAH) to select relevant journals themselves. We recommend monitoring as broad a range of local sources as possible not to miss any relevant ICSRs. The sources we monitor include subscribed to and delivered via post), electronic journals (accessed online with or without subscription, or delivered to us via e-mail), local databases, and websites. This comprehensive approach ensures that all pertinent safety information is identified and reported on time in compliance with pharmacovigilance requirements.
-
At what frequency should local literature be monitored?
According to GVP Module 6 VI.B.1.1.2, literature should be monitored at least once a week to maintain awareness of any potential safety information related to the medicinal product. This frequent monitoring ensures that any new safety signals or emerging safety issues are promptly identified and reported.

