January 11, 2022
Marketing Authorization Transfer Considerations
Overview
The high level of Mergers and Acquisitions (M&A) reflects the incredibly dynamic nature of the Biopharmaceutical industry today. Waves of mergers and acquisitions have resulted in major consolidation during the previous few decades. Pharmaceutical businesses perform more acquisitions and larger transactions than companies in other industries, including the tech industry.
Mega-deals and bolt-on acquisitions are both critical components of the biopharma business strategy for generating growth and gaining a competitive advantage. Mergers and acquisitions in the pharmaceutical industry benefit both large corporations and small biotech companies. Large corporations are rapidly expanding their R&D pipelines, product portfolios and increasing a potential profit. Emerging biotech enterprises receive a successful exit option or the chance to expand their brand awareness into a new market.
Large transactions of M&A, on the other hand, entail a complicated Marketing Authorization Transfer (MAT) process, in which business and regulatory compliance are essential factors. The MAT process results in the original Marketing Authorisation Holder’s rights and duties for the specific medical product being transferred to the new Marketing Authorisation Holder on a defined date when the transfer is achieved.
MA transfer can only be initiated once a MA has been granted. Existing authorizations must have a remaining validity time of more than three months for products that have already been approved. Before the transfer application may be handled, the existing Marketing Authorisation must be renewed if the time is shorter than three months. It is important to emphasize, that the pharmaceutical product granted to the new Marketing Authorization Holder is authorized under the same criteria as before:
- It must be produced and monitored in accordance with the approved dossier;
- Any post-authorization responsibilities, such as data lock points in the Periodic Safety Update Report, follow-up measures, and specific obligations, must be met as before;
- Its expiry date is the same, and hence the date by which it must be renewed in order to be valid, if applicable;
- To avoid the expiration of the Marketing Authorization, the product must be placed on the market within three years after the original approval date in the Member State where it is authorised (“Sunset Clause”).
Fundamental challenges
However, in addition to the above-mentioned benefits that M&A brings to businesses, there are extra challenges that need to be overcome. Here are some of the most fundamental challenges that might arise throughout the development and implementation of a successful MAT process.
- Effective date. Implementation
Why it is important to set an effective date (implementation date)?
When a Marketing Authorization is transferred, the new MAH receives both rights and duties. The implementation date is the date on which the Transferee takes over ALL responsibilities as the Holder of the MA. It is critical that all stakeholders, including the present and future MAHs, as well as the competent authority concerned, be aware of the date on which the new Marketing Authorisation Holder takes all of the responsibilities and obligations for the medical product. As a result, several competent authorities need a “waiver declaration” from the current Marketing Authorization Holder, in which the current MAH cedes its rights to the new MAH beginning on a specific date.
Implementation of the Marketing Authorisation Transfer, i.e. selling the medicinal product with the new Marketing Authorisation Holder’s name in the product information texts, may normally be done by following approval from the Marketing Authorisation Transfer application’s effective date. It is generally possible for the effective date to be determined by agreement between the current and proposed Marketing Authorisation Holders and the competent authority concerned and therefore it is important for it to be clearly stated.
Noteworthy:
When many Member States are involved, such as for an MRP/DCP product, the disparity in timelines for the Member States, ranging from instantaneous upon receipt of the application to 6 months, complicates planning the implementation following approval. This means that when a product is transferred across the multiple Member States, very clear contractual arrangements must be agreed upon between the current and future MAHs, delineating the various responsibilities during the transitional period, such as batch release and submission of regulatory activities, such as variations, because the transfer will not be completed in all Member States concerned at the same time.
When the new MAH takes over the entire responsibilities, it is important to consider a timeline that is reasonable, while choosing an implementation date for market adjustments to go into effect, in order to prevent companies from being left without supplies (out-of-stock), and of course neither the patient should be subjected to an interruption of ongoing therapy as a result of administrative changes that have no bearing on quality, effectiveness, or safety. When setting the effective date (implementation date) keep in mind that the timetable for approval varies from one Member State to the next.
For the Transfer of a Marketing Authorisation covering medicinal products already marketed by the Transferor, the proposed date should be set taking into account the following timelines:
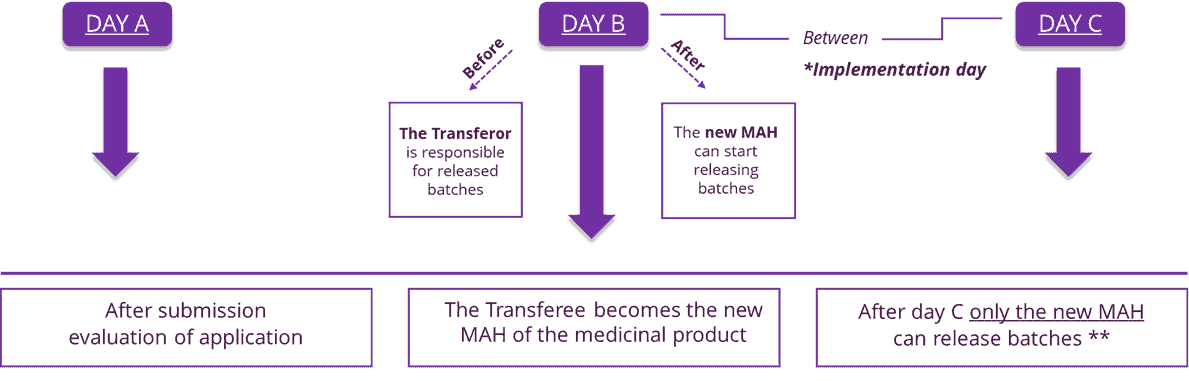
* Information as to the date on which the Transferor will release the last produced batch in the distribution chain might be required to be included in the Transfer application.
** There are exceptions, in some Member States, batches that were placed on the market before Day C and contain the name of the previous MAH are permitted to remain on the market.
- Grace period
Will there be product supply in the new markets once the MAT is completed?
One of the most essential factors in the MA transfer of drug rights is a grace period, which is the period when the original holder of the registration certificate can sell-out medicine before his license is entirely transferred to the new holder. Product supply may need to be built up in order to maintain a market presence throughout the transition phase, necessitating a grace period for current product sales in the seller’s livery following the transfer. Due to production schedules, capacity, or other obstacles like raw materials on backorder, it is not unusual for acquiring firms to have a three- to six-month delay or more. Acquiring firms may be able to alleviate these delays by implementing robust manufacturing risk management procedures. Assessing the situation, some companies release more pharmaceuticals before receiving confirmation of the transfer, while others in advance negotiate with National authorities on the granting of a grace period. It is important to note, that the grace period should be agreed in advance with the Agency in the Member States before the MAH transfer takes place, to avoid supply disruption in the future. In all scenarios, companies should have a brief plan and be prepared for the transition.
What are the MAT timelines in EU?
EU countries have distinct timetables that must be taken into account for the overall transfer strategy. The timeline refers to the time that is necessary for a Marketing Authorisation Transfer application to be assessed and approved before the transfer may take place. It is important to avoid assuming a relationship between the amount of documentation necessary, the evaluation, and the timetable.
For products approved by Centralised Procedures, Mutual Recognition/Decentralized Procedures, and National Procedures, the requirements and timelines for MAH transfer vary. The rules and timings for MAH transfer differ by count for items authorized via MRP/DCP or National process. In the majority of Member States, the Marketing Authorisation transfer takes between 1 to 2 months, but in some Member States, it varies significantly from immediate implementation upon receipt of the application to three or more months. It is vital to look at the timelines for submissions and clearances from authorities to make sure the supply chain does not get disrupted.
Marketing Authorization transfer approximately takes from 1 to 2 months.
Pharmacovigilance (PV) System
One of a Marketing Authorization holder’s most important responsibilities is to “operate a pharmacovigilance system for the fulfilment of his pharmacovigilance tasks” and to “have permanently and continuously at his disposal an appropriately qualified person responsible for pharmacovigilance” who is “responsible for the establishment and maintenance of the pharmacovigilance system”. Each Marketing Authorisation Holder has its own pharmacovigilance system. During a MA transfer, another alteration that frequently occurs is a change in the pharmacovigilance system.
Below listed additional elements which can be updated during a MA transfer process of the pharmacovigilance system:
- Pharmacovigilance System Master File (PSMF) declaration;
- Risk Management System;
- Qualified Person for Pharmacovigilance (QPPV).
A change to element(s) to the summary of the pharmacovigilance system master file (PSMF), e. g. the Qualified Person for Pharmacovigilance (QPPV) or of the PSMF location resulting from the transfer of the Marketing Authorisation (MA) in some of Member States can be notified as part of the transfer application without the need for a separate variation.
The summary of the transferor’s pharmacovigilance system in the MA dossier needs to be replaced in the transfer application with an updated summary of the transferee’s pharmacovigilance system including:
- a proof that the transferee has at his disposal a QPPV, the Member State(s) in which the QPPV resides and carries out his/her tasks and its contact details;
- a statement signed by the transferee to the effect that the applicant has the necessary means to fulfil the tasks and responsibilities;
- a reference to the location where the PSMF for the medicinal product is kept;
- a nomination of a Local Person for Pharmacovigilance (LPPV / Local QPPV) of the transferee may be required by some Member States during the MAT.
It is nevertheless required to update accordingly the information in the Article 57 database after the conclusion of the procedure for the MA transfer.
It is important, that a detailed Pharmacovigilance Agreement must be created as soon as a transaction is completed. PV-related roles and duties must be clearly defined. The Marketing Authorization Holder is always in charge of the final decision.
- Pricing & Reimbursement.
It is important to emphasize, that the new Marketing Authorization Holder should take into consideration declared prices, reimbursement conditions, confidential price and supply related commitments in the territories. In some of Member States, following a MAT for reimbursed medicines, there are requirements to notify about new MAH and their contact person to relevant Authorities within certain timelines. In case of any managed entry agreements (MEA), they might require re-signing by new MAH. National procedures and timelines must be considered if a new MAH seeks to alter pricing.
- Other procedures. Submission of additional changes
The Marketing Authorisation Transfer procedure involves a new MA Holder (legal entity) taking over responsibility for a specific medicinal product, resulting in changes to the Marketing Authorisation Holder’s information (name and contact details), which are the only changes in the approved dossier and product information texts that are a direct result of The Marketing Authorisation Transfer. On the other hand, a MAT procedure is frequently accompanied/followed by a variety of prospective adjustments.
Changes of Manufacturers and Local presentative.
The manufacturer may change as a result of the Marketing Authorization Transfer. The modification might be the inclusion of a new manufacturer, the deletion of an existing one, or the replacement of an existing one with a new one, for either the active ingredient or the final product. As a result, additional processes may be necessary.
Furthermore, it is critical to distinguish which processes are the duty of the existing MAH and which should be undertaken by the prospective MAH. It is critical to understand which modifications affect package leaflets, labelling, and packaging, as well as whether the changes can be implemented before submission to authorities or just after receiving clearance.
Medicinal product name change.
As a result of the Marketing Authorization Transfer, the medical product’s name may change. This could be a necessary change because the product name includes the Marketing Authorisation Holder’s name, or because the new MAH wants the product to have a different invented name. In order to change the name of a medicinal product, a variation is required and should be submitted separately and in parallel to the transfer procedure. In the case, the transfer procedure concerns a medicinal product whose name is constructed as [INN / common name + name of the MAH], the name of the medicinal product needs to be changed to reflect the name of the new MAH (transferee).
Documents preparation and submission.
Requested information and documents for the MAT process can be different in each Member States, according to National requirements. Documentation preparation for Marketing Authorization Transfer requires a lot of knowledge and attention. It is important to prepare documentation correctly, to avoid any necessary potential future mistakes, which can slow down or delay the MAT process. The regulatory specialist should plan out all of the documents that will be required, as well as how much time each item will take to complete. The situation becomes more complicated as more pharmaceutical items, Member States, and processes are involved in the Marketing Authorization Transfer for which variations must be submitted.
Some documentation, like regulatory forms and certificates, may be completed quickly, whilst others, such as product artwork preparation and Certificates of Pharmaceutical Product, might take even a few months to get. If the revisions involve updates to the product information texts (for example, changes to the Marketing Authorization number, local representative, product name, and/or manufacturers), the regulatory actions should be well-planned to minimize repetitive submissions that alter the texts.
In order to avoid a variety of risks, delays and guarantee a successful MAT process, it is recommended to use Regulator Specialists help during the MA transfer. They will ensure the fluent process by preparing documentation, communicating with Member States agencies and executing transactions without disruptions and unnecessary additional costly procedures.
Please don’t hesitate to contact us if you have any questions regarding the Marketing Authorization Transfer process.
Back to news