May 11, 2023
ISO IDMP: Current Status and Future Developments
As of 2015, EMA has taken a challenge to be the first Agency, that plans to implement.standards agreed on ISO for identification of medicinal products (ISO IDMP). Considering the number of countries, products, different types of procedures (central and national), systems to be affected, it is a giant and complex task.
ISO IDMP implementation is also known as Substance Product Organization Referentials (SPOR) system at EMA. Despite the complexity of the task, the implementation of the system moves forward and many milestones are already achieved [Figure 1].
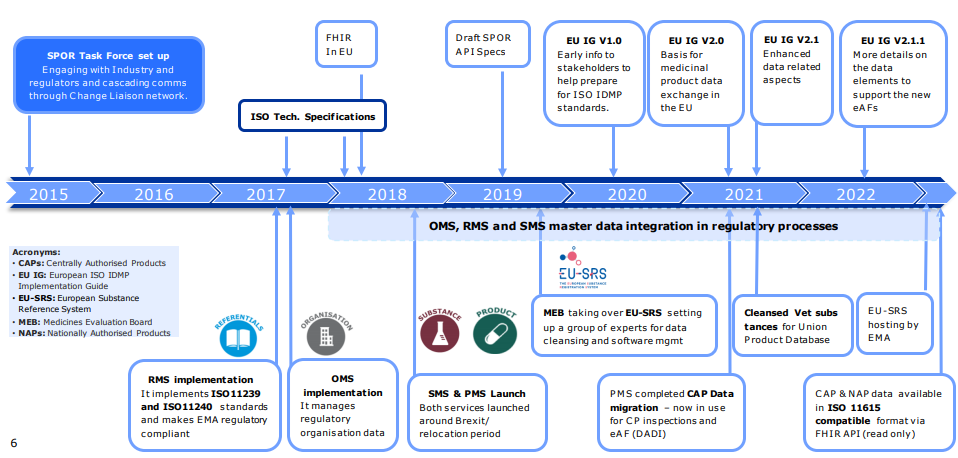
Figure 1. Timeline of SPOR task force and already achieved milestones [1]
Here we would like to summarize the latest updates and the most upcoming activities which could be expected as well as impacts for MAHs.
SPOR implementation is based on data-centric target operating model (TOM), which.means that EMA tries to set up a system where data can be reused in as many procedures as needed. Reuse of data will facilitate filling and checking data for different forms for different purposes and allow to avoid human mistakes. Even now SPOR system is very broadly used in different regulatory processes [Figure 2] and it will be used even more broadly in the future.

Figure 2. SPOR integration in Regulatory processes through Product Lifecycle [1]
One of the components of SPOR is PMS, which implementation and integration is a hot topic nowadays. As it may be seen from the Figure 1, PMS together with SMS were launched in 2018. However, both components are not available for industry directly, at least for now. EMA already completed data migration from SIAMED database (internal EMA database for CAP products) and xEVMPD database for CAP products to PMS. The migration is ongoing for NAP products. xEVMPD and PMS are synchronized systems and the product update done in xEVMPD affect the data in PMS already. MAHs are encouraged to analyse EU IG chapter 7 Migration guide, in order to understand how migration is done and how the product data shall be organized that it would be correctly migrated. PMS will serve as Art 57 database in the future.
Even though EMA has xEVMPD and SIAMED databases the IDMP standards require much more data elements about the product, which already are not tracked along with the product. The additional data elements will serve for multiple purposes in different processes during the product lifecycle. MAHs are encouraged to analyse EU IG chapter 2 for data elements, which will be required to fill by MAHs to comply with IDMP standards. When it will be required, it is still unknown. At some point, EMA will announce the period of enrichment of product data.
During 2022, EMA mostly focused on web-based forms development primarily for variations of already existing marketing authorizations. Web-based forms were activated for CAP authorizations on 4th of November 2022 and it is planned to be implemented for all NAPs later on 2023. There is still not mandatory to be used yet as transition period is not announced. Web-based forms will change electronic application form (eAF).
New platform – Product Lifecycle Management Portal (PLM Portal) for filling web-based forms were presented by EMA at the beginning of November 2022. The PLM Portal is dedicated only for forms filling and extractions, while there are no changes in pathways of submission. Three platforms are integrated with each other: the product data are stored in PMS, where industry does not have access. However, data, which is present in PMS, may be visible via PLM portal (only for CAPs for today). If MAH would like to change/correct some data elements of product, it shall be done via xEVMPD.
It is interesting that PLM portal has a forum, where official EMA communication flow as well as where other users may discuss different topics and help each other on arising issues.
As PLM Portal does not serve for any submissions, it can be used as a sandbox to try different functionalities and understand how the system works. To access PLM Portal, the potential user has to request and be confirmed for one of the dedicated.roles (e. g. eAF Applicant Manager, EAF Applicant Coordinator, etc.) for PLM Portal via EMA Account Management.
The pdf extracted from PLM Portal contains invisible xml file in HL7 FHIR format. HL7 FHIR format is the new format for ISO IDMP compatible data submissions which will replace current eXtended EudraVigilance Product Report Message (XEVPRM) format. HL7 FHIR format will be implemented by FDA and other authorities as well. Thus, in the future the worldwide system would allow us not only to have data on the same standards.but also to exchange them in the same formats when it would be required.
You may see in Figure 3 the most upcoming activities on PMS development announced by EMA. In blue color you may see confirmed activities and in the red color suggested but not already confirmed ones. EMA plans to migrate all xEVMPD data to PMS and made available web-based forms for NAPs within Q2 of 2023,.create Product User Interface in PMS within end of 2023. Along with these activities electronic Product information portal is developed.

Figure 3. PMS activities roadmap [1]
It may be seen that a lot of activities and developments are ongoing at EMA side, but what needs to be done from MAH perspective?
Officially EMA request from MAHs the following preparations:
- align their systems with the released terminologies for referentials, organisation and substance data;
- request the registration of any new or missing controlled vocabulary required for the submission of medicinal product data;
- start structuring their product data according to the rules described in chapter 2 of the EU IG.
However, we would suggest doing more:
- try PLM portal when able to do it, to get familiar with the system;
- check migration rules and make adjustments in xEVMPD database if mistakes are identified already;
- follow announcements and evolution of SPOR systems via EMA communication channels;
EMA is transparently sharing activities which are ongoing and quarterly system demos show the implemented improvements publicly. Thus industry, at least the bigger pharma companies, can take part as a developer,.not only accept the upcoming changes as all inputs and feedback for improvements are encouraged.
On the other hand, considering the number of developments, sometimes it is even difficult.to follow the changes and expectations of regulators for smaller pharma companies. Thus, if you would like to know more about IDMP and how we can help you please contact us.
[1] Presentation – European Union (EU) International Organisation for Standardization (ISO) for identification of medical products (IDMP)/Substance, Product, Organisation and Referential (SPOR) data Task Force meeting – January 2023 (europa.eu)
Back to news